cobalt electron configuration|What is the s,p,d,f configuration of Co? + Example : iloilo Learn how cobalt atoms arrange their 27 electrons in different orbitals and subshells, and how this affects their valence and valency. Find out the shorthand notation, the . dr.dk: TEKST-TV side 159 - DR1
cobalt electron configuration,Mar 23, 2023 Learn how to write the electron configuration of cobalt (Co) through orbit (Bohr model) and orbital (Aufbau model). Compare the differences and similarities between the two methods and see examples and diagrams.What is the s,p,d,f configuration of Co? + Example There are 27 electrons for the Cobalt atom. When we write the configuration, we'll put all 27 electrons in orbitals around the nucleus of the Cobalt atom. In this video we'll use .Learn how to write the electron configuration for cobalt (element 27) in ascending order of orbital energies and levels. See the orbital diagram, valence electrons, quantum numbers and .

Learn how cobalt atoms arrange their 27 electrons in different orbitals and subshells, and how this affects their valence and valency. Find out the shorthand notation, the .
cobalt electron configuration Learn how cobalt atoms arrange their 27 electrons in different orbitals and subshells, and how this affects their valence and valency. Find out the shorthand notation, the .
Cobalt is a transition metal with symbol Co and atomic number 27. Its electron configuration is [Ar] 3d 7 4s 2, with 2 or 9 valence electrons.
Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the .Learn the electron configuration of cobalt, a ferromagnetic metal with a bluish and white hue, and its isotopes, precautions and biological function. The web page also provides the covalent and .

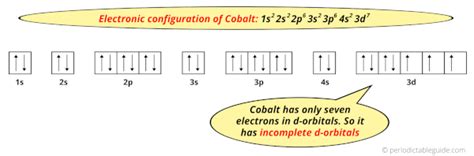
Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and therefore has 7 d electrons . What is the Electron Configuration of Cobalt? The electron configuration of the Cobalt can be represented as the [Ar] 3d7 4s2 in its most accurate and precise form. This electron configuration of the element . The s,p,d,f configuration for cobalt (Co) is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7, determined by the position of the element on the periodic table. Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and therefore has 7 d electrons d^7. The element cobalt can be found in the 4th row .Electronic configuration of the Cobalt atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2 Reduced electronic configuration Co: [Ar] 3d 7 4s 2. Below is the electronic diagram of the Cobalt atom Distribution of electrons over energy levels in the Co atom 1 .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .
La configuration électronique du cobalt est 1s2 2s2 2p6 3s2 3p6 3d7 4s2. Le cobalt est un élément chimique du tableau périodique, il se situe dans le groupe 9, son symbole est Co et son numéro atomique est 27. Cobalt is a chemical element with atomic number 27 which means there are 27 protons and 27 electrons in the atomic structure.The chemical symbol for Cobalt is Co. Electron Configuration and Oxidation States of Cobalt. Electron configuration of Cobalt is [Ar] 3d7 4s2. Possible oxidation states are +2,3. Electron Configuration
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers .
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid all this if you use our . Thus, the electron configuration for Cobalt at ground state would simply be Co: [Ar] 4s 2 3d 7. The reason why it is 3d 7 can be explained using the periodic table. As stated, you could simply count the boxes on the periodic table, and since Cobalt is the 7th element of the first row transition metals, we get Co: [Ar] 4s 2 3d 7.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Cobalt is [Ar] 3d7 4s2. Possible oxidation states are +2,3.
cobalt electron configuration What is the s,p,d,f configuration of Co? + Example Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at .
Therefore, the complete electron configuration of cobalt (Co 3+) ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 and the unabbreviated electron configuration of cobalt (Co 3+) ion is [Ar] 3d 6. Also, this electron configuration shows that the cobalt (Co 3+) ion has three shells and the last shell has fourteen electrons. The charge of the element changes .Complete ground state electronic configuration for the Cobalt atom, Unabbreviated electronic configuration. 1s2 2s2 2p6 3s2 3p6 3d7 4s2. Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule. As per the Aufbau principle the electrons will occupy the orbitals .钴的电子排布为 1s2 2s2 2p6 3s2 3p6 3d7 4s2。 钴是元素周期表中的一种化学元素,位于第 9 族,符号为 Co,原子序数为 27。La configuration électronique du cobalt est [Ar] 3d7 4s2. Les états d’oxydation possibles sont +2,3. Les états d’oxydation courants du cobalt comprennent +2 et +3, bien que des composés avec des états d’oxydation allant de -3 à +5 soient également connus.Cobalt. Full electron configuration of cobalt: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2 iron ← cobalt → nickel. © 2009-2016 | www.prvky.com | kontaktkontakt The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. Cobalt is a very hard, lustrous bluish grey metal that belongs to d-block and is under the category of transition metals. It is a hexagonal closed pack (hcp) crystal structure that is solid in normal conditions and is isolated by the process of smelting .
cobalt electron configuration|What is the s,p,d,f configuration of Co? + Example
PH0 · What is the s,p,d,f configuration of Co? + Example
PH1 · Electron configuration for Cobalt (element 27). Orbital diagram
PH2 · Electron Configuration of Cobalt 【Electron Configuration】 2022
PH3 · Electron Configuration for Cobalt (Co and Co2+, Co3+)
PH4 · Electron Configuration for Cobalt (Co and Co2+,
PH5 · Electron Configuration Chart of All Elements (Full Chart)
PH6 · Cobalt Electron Configuration: Distribution of Electrons in Shell
PH7 · Cobalt Electron Configuration (Co) with Orbital Diagram
PH8 · Cobalt (Co)
PH9 · Cobalt
PH10 · A step